Exosome Therapy For Hair Loss
Exosome Hair Loss Therapy
Exosome Therapy is a promising novel hair loss treatment modality that we have introduced into our practice. It is recommended in conjunction with other therapeutic treatments such as minoxidil, finasteride, low level laser therapy, and other treatments.
What are Exosomes?
Exosomes are extracellular vesicles that are rich in growth factors, cytokines, microRNA and mRNA which all help to promote hair growth. They can increase hair density and shaft diameter, reactivate miniaturized hairs and generate new hair growth. These vesicles modulate cell communication causing cell response for differentiation, development and growth.
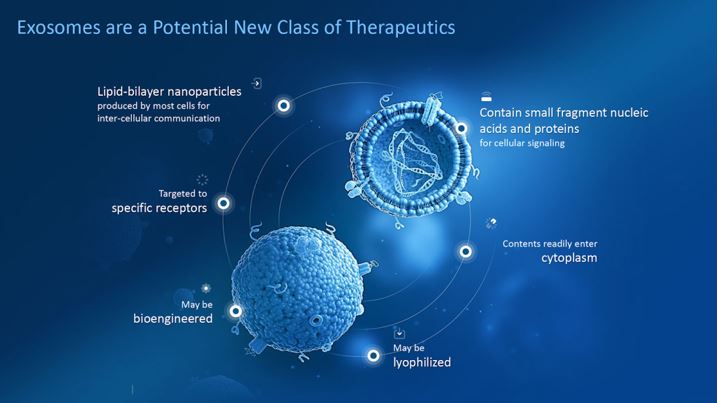
Photo: https://capricor.com/exosomes/
 The product used in our office is Exovex which is mesenchymal stem cell-derived acellular growth factors from human placenta. They are sterilized in a cGxP compliant laboratory and undergo strict screening processes to ensure that they are free from any communicable diseases such as HIV, Hepatitis C, Covid, etc.
The product used in our office is Exovex which is mesenchymal stem cell-derived acellular growth factors from human placenta. They are sterilized in a cGxP compliant laboratory and undergo strict screening processes to ensure that they are free from any communicable diseases such as HIV, Hepatitis C, Covid, etc.
Exosomes have been utilized in many different medical treatments including cardiovascular diseases, burns, wound healing, joint diseases and hair loss.
How is the procedure performed?
The procedure takes about 30 minutes. You will be seated in a comfortable chair, where we will clean the skin and then inject some numbing medication (lidocaine). 12 billion vesicles are then injected into the scalp, focusing on areas of most hair loss.
What are the benefits of exosome injection therapy for hair loss?
- Stimulation of hair growth: The growth factors and signaling molecules in exosomes can stimulate the growth of new hair follicles and promote the restoration of hair growth in areas where hair has been lost.
- Non-invasive procedure: Unlike hair transplantation surgery, exosome injection therapy is a non-invasive procedure that can be performed quickly and with little discomfort.
- Potential for long-lasting results: Exosome injection therapy has the potential to produce long-lasting results, as the growth factors and signaling molecules in exosomes can continue to promote hair growth for an extended period of time.
- Reduced risk of side effects: Exosome injection therapy carries a reduced risk of side effects compared to other treatments for hair loss, such as topical or oral medications, as it is a relatively new and minimally invasive procedure.
What is the recovery process?
We ask that you refrain from vigorous exercise for 24-48 hours after the procedure to decrease risk of swelling. You may shower as usual and return to your normal hair care routine the morning after the procedure. You may return to work the same or next day.
When will I see results?
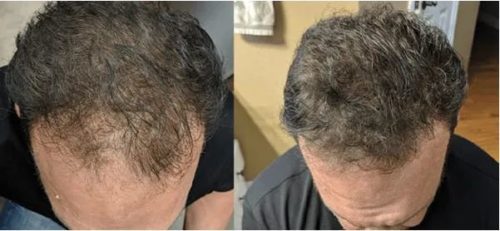
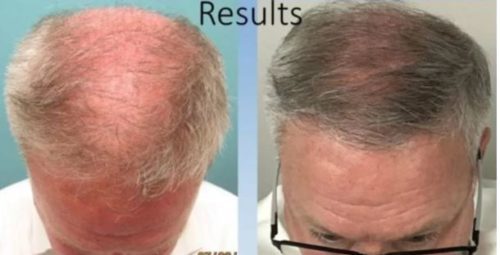
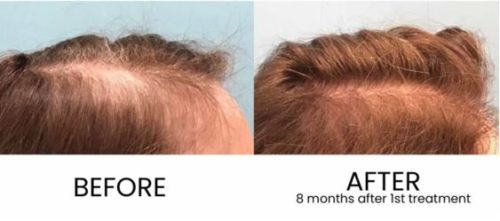
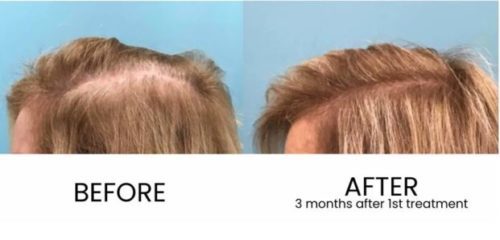
Results can be seen in as little as 30 days and are thought to last for up to 3 years. Results vary based on many factors, but we do everything in our power to maximize results.
What has the research shown?
There are new studies on the horizon. Several preliminary studies have found that this evolving treatment is safe for use with promising results. It is not currently FDA approved yet, but is FDA registered and manufactured under strict controls.
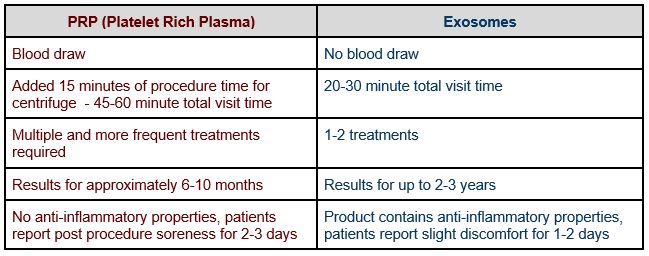
What is the difference between Exosomes and PRP?
PRP (Platelet Rich Plasma) requires a blood draw usually from your arm which is then centrifuged to separate out the growth factors, and re-injected into the scalp. Exosomes are exogenous (from a source outside of your body), so a blood draw is not necessary.
Current Relevant Articles
Ajit, A., Nair, M. D., & Venugopal, B. (2021). Exploring the Potential of Mesenchymal Stem Cell–Derived Exosomes for the Treatment of Alopecia. Regenerative Engineering and Translational Medicine, 7, 119-128.
Kost, Y., Muskat, A., Mhaimeed, N., Nazarian, R. S., & Kobets, K. (2022). Exosome therapy in hair regeneration: A literature review of the evidence, challenges, and future opportunities. Journal of Cosmetic Dermatology, 21(8), 3226-3231.
Sasaki GH. Clinical Use of Extracellular Vesicles in the Management of Male and Female Pattern Hair Loss: A Preliminary Retrospective Institutional Review Board Safety and Efficacy Study. Aesthet Surg J Open Forum. 2022 May 24;4:ojac045. doi: 10.1093/asjof/ojac045. PMID: 35923863; PMCID: PMC9342625.